We know that Scientists
Predict
Observe
Record
We are doing an experiment with
Milk
Food Colouring
Dish washing liquid
Droppers
Toothpicks
Before we start our experiment we are going to predict what happens
I think it will make sticky stuff and lots of bugs will stick to it - Bryn
I think it will bubble up and it will have lots and lots of colours mixed up together - Tiaan
I think it will bubble up into a colourful chemical reaction - Riley B
I think it will bubble up and get all over us - Ashley
I think it will turn into potions - Grace
I think it will explode like a volcano - Emma
Now it's time to test it out
First
we put milk into our plate
then we put drops of colour into the milk
Finally we add little bits of dish washing liquid on a tooth pick
What Happened???
when we were putting the dishwashing liquid into the bowl where the food colouring was it kind of like exploded. - Tiaan
when we put the food colouring in it was really hard because we couldn't move the plate at all- Nethasa
when we put our squirter in it changed all around the plate - Ashley
Here are some photos
Why does it happen?
Milk
is mostly water, and water has a property called surface tension, this
is because all the water molecules are strongly attracted to other
water molecules, but not to air, so they try to get away from the
surface of the drop, making the surface as small as possible, this is
why raindrops are approximately spherical - the shape with the least
surface for its volume.
This means that the surface of water, or milk, is always trying to shrink.
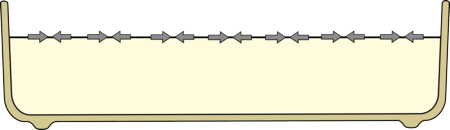 |
The surface of the milk is always trying to shrink, due to surface tension.
|
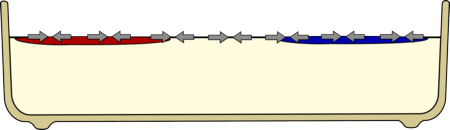 |
The food colouring is less dense than the milk so it floats on the surface.
|
Something
else you may have noticed is that the food colouring seems to float on
the surface of the milk, this is because the milk has lots of
substances dissolved in it such as Calcium making it more dense than
the food colouring which is almost entirely water.
Washing up liquid is designed to break up the
surface tension so water can dissolve fats and grease. This means that
where you add the washing up liquid the surface tension is much weaker
than everywhere else, so this surface gets hugely stretched by the milk
which hasn't met the washing up liquid yet.
 |
The
washing up liquid breaks the surface tension where it lands, allowing
this surface to stretch hugely, causing the rest of the surface to
shrink hugely.
|
 |
This shrinkage pushes the food colouring downwards, and it floats up again forming beautiful patterns.
|
Because
the rest of the surface is shrinking it must be getting thicker, this
pushes the food colouring downwards, and there is a current below the
surface flowing back towards the washing up liquid pulling the food
colouring along. It then floats back up to the surface producing
beautiful patterns.
Why does washing up liquid reduce surface tension?
A washing up liquid molecule is made up of a water
loving head and a water hating tail, so when you add it to water the
molecules arrange themselves over the surface - head inwards. The water
is strongly attracted to the heads of these molecules, so is now stops
trying to reduce its surface area, and the surface tension is far
weaker.

















No comments:
Post a Comment